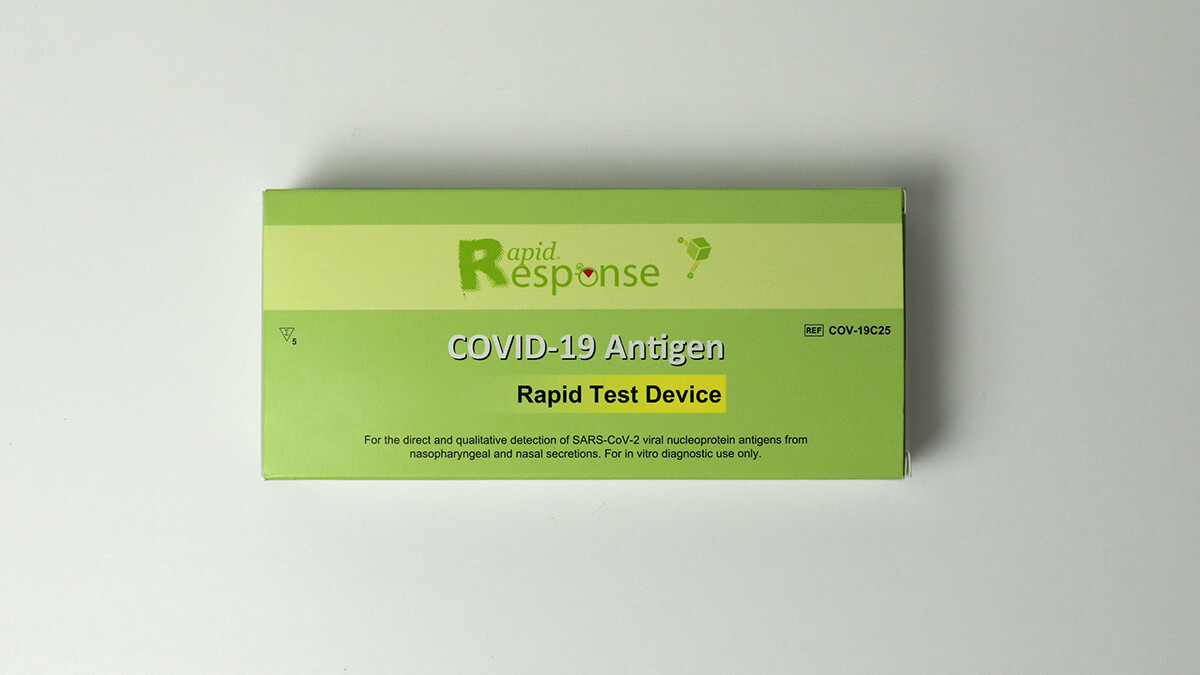
Shop Rapid Tests
Additional Information
The BTNX SARS-CoV-2 Viral Nucleoprotein Antigen test has a 90+% sensitivity and 100% specificity if completed with a shallow nasal swab. Results are available for patients within 6 days of symptom onset.
Contents
Individually packed test devices, extraction buffer, extraction tube, nozzle with filter, tube stand, individually packed swabs, package insert. A Test Application Guide (digital instructions) will be provided by email with each order.
Downloadable instructions
Video instructions
Further Details
The BTNX COVID-19 Antigen Rapid Test Device is an in vitro immunochromatographic assay for the direct and qualitative detection of SARS-CoV-2 viral nucleoprotein antigens from nasal and nasopharyngeal secretions from individuals suspected of COVID-19 within 6 days of symptom onset. Results are for the identification of SARS-CoV-2 viral nucleoprotein antigen. Antigen is generally detectable in nasopharyngeal and nasal secretions during the acute phase of infection. Positive results indicate the presence of viral antigens, but clinical correlation with patient history and other diagnostic information is necessary to determine infection status. Positive results do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease. Laboratories are required to report all positive results to the appropriate public health authorities. Negative results should be treated as presumptive, and do not rule out SARS-CoV-2 infection and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions. Negative results should be considered in the context of a patient’s recent exposures, history, and the presence of clinical signs and symptoms consistent with COVID-19, and confirmed with a molecular assay, if necessary, for patient management.
Pursuant to section 5 of the Interim Order Respecting the Importation and Sale of Medical Devices for Use in Relation to COVID-19, made by the Minister of Health on March 18, 2020, the BTNX Rapid Response™ COVID-19 Antigen Rapid Test Device is now authorized for sale or importation in Canada.
Sample: Shallow Nasal
Format: Cassette
Time to result: 15 minutes
Storage Condition: 2°C – 30°C/36°F – 86°F